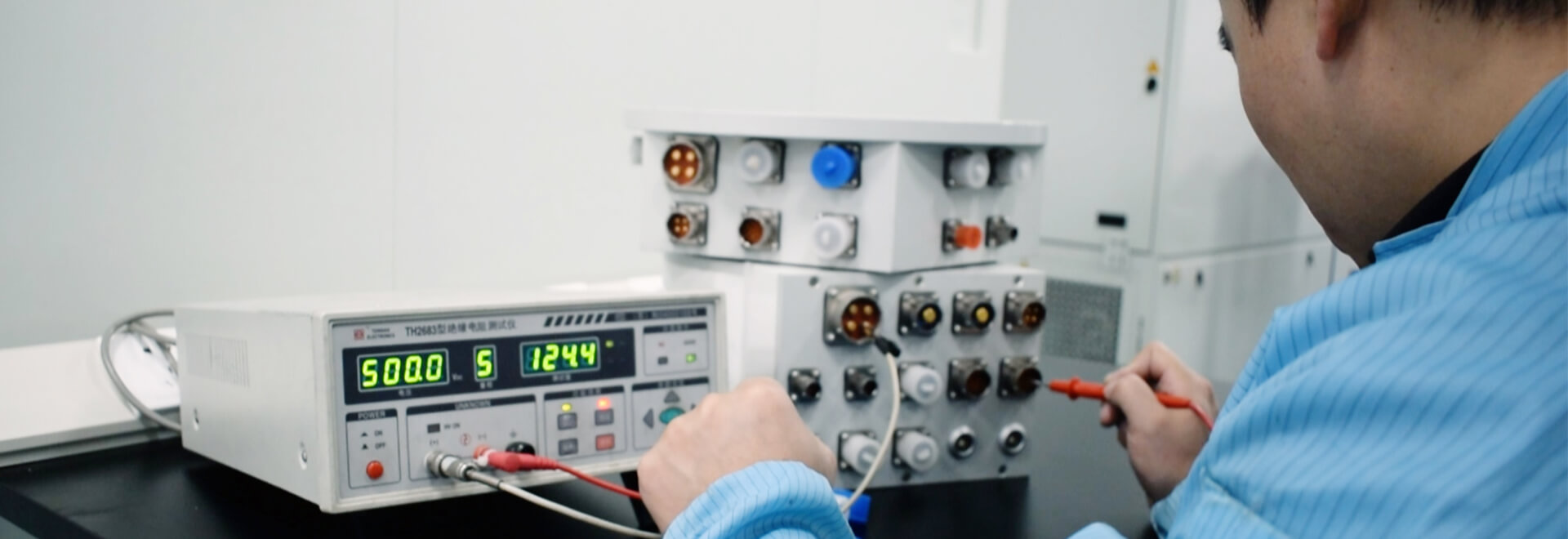
In today’s rapidly advancing healthcare landscape, the importance of Electromagnetic Compatibility (EMC) in medical devices cannot be overstated. For medical equipment to operate safely and effectively, particularly in high-stakes environments like operating rooms, diagnostic imaging departments, and intensive care units, every component must function seamlessly. This includes essential parts like slip rings, which are crucial for enabling the smooth transmission of electrical signals between stationary and rotating parts of medical equipment. However, as medical technologies evolve, the need for stronger EMC compliance is growing, especially with the rise of wireless devices, AI-powered diagnostics, and complex hospital environments. At Grand Slip Ring, we understand the critical role that EMC compliance plays in patient safety and the efficiency of healthcare operations. In this article, we’ll explore the key EMC requirements that should guide your purchasing decisions, the direct impact of EMC on hospital operations and patient safety, and how partnering with the right manufacturer—like Grand Slip Ring—can ensure reliable, long-term performance for your medical devices.
Why EMC Matters to Medical Slip Ring Buyers
EMC Defined: The Invisible Guardian of Medical Systems
Electromagnetic Compatibility (EMC) ensures that medical slip rings can operate reliably in complex electromagnetic environments, without generating harmful interference to other devices or being disrupted by external interference. For medical buyers, EMC is not just a technical term but a critical safeguard for patient safety and device functionality.
Real-World Risks of Non-Compliance
- Patient Safety Threats: A single electromagnetic interference (EMI) event in a hospital could disrupt life-saving equipment. For example, EMI from a non-compliant slip ring might cause a surgical robot to malfunction during a procedure, leading to delayed responses or incorrect actions.
- Operational Disruptions: Hospitals rely on continuous operation of imaging systems (e.g., MRI, CT scanners) and patient monitors. EMC failures in slip rings can trigger false alarms, data corruption, or even system downtime, costing thousands of dollars per hour in lost productivity.
- Legal and Financial Liabilities: Regulatory bodies like the FDA and EU MDR mandate strict EMC compliance. Non-compliant devices risk recalls, fines, and reputational damage. In 2024, a leading manufacturer faced a $2M FDA penalty due to EMI-related failures in cardiac monitors.
The Unique Role of Slip Rings in Medical Devices
Medical slip rings are the “nerve centers” of rotating equipment, enabling seamless power and signal transmission in applications such as:
- Surgical Robotics: High-precision movements require zero latency and immunity to EMI from nearby electrosurgical tools.
- Imaging Systems: MRI and CT scanners demand ultra-low noise to maintain image clarity; even minor EMI can distort diagnostic results.
- Patient Monitoring: Slip rings in wearable or bedside monitors must resist interference from wireless devices (e.g., Wi-Fi, Bluetooth) to ensure accurate vital sign tracking.
Buyer Awareness Gap: Beyond Basic Compliance
Many buyers focus solely on certifications like IEC 60601-1-2 but overlook real-world scenarios. For instance:
- Dynamic Environments: Operating rooms (ORs) with multiple wireless devices create unpredictable EMI patterns. A slip ring passing lab tests might fail in a crowded OR.
- Long-Term Stability: Materials like low-magnetic alloys and shielding coatings degrade over time. Buyers need lifecycle data to ensure sustained EMC performance.
The Cost of Ignorance: Case Studies
- A hospital in Germany reported a 30% increase in ECG monitor errors traced to EMI from aging slip rings in adjacent MRI systems, requiring a $500K system overhaul.
- A U.S. surgical robot manufacturer avoided a potential recall by upgrading to EMC-optimized slip rings during pre-market testing, saving an estimated $1.2M in compliance costs.
Regulatory Landscape: A Moving Target
Standards like IEC 60601-1-2:2020 and China’s YY9706.102-2021 now emphasize real-world performance validation over traditional lab tests. Buyers must verify that suppliers test slip rings in simulated hospital environments, not just controlled labs.
Key Takeaways for Buyers:
- EMC directly impacts patient outcomes, operational efficiency, and legal compliance.
- Prioritize suppliers offering scenario-based testing (e.g., OR simulations) and transparent lifecycle data.
- Proactive EMC investment reduces long-term risks and Total Cost of Ownership (TCO).

Key EMC Requirements Should Prioritize
When purchasing medical slip rings, it’s crucial for consumers to understand the key EMC (Electromagnetic Compatibility) requirements that can directly impact the performance and safety of their equipment. These requirements ensure that the slip rings can operate without causing harmful interference to other devices or being vulnerable to external electromagnetic disturbances. Here, we will explore the three primary EMC requirements that buyers should prioritize when selecting medical slip rings:
Emission Control
Objective: Preventing electromagnetic “noise pollution” in sensitive hospital environments.
Medical environments, such as operating rooms (OR), intensive care units (ICU), and diagnostic rooms, are full of critical electronic devices that rely on clear signals and stable operations. For example, ECG monitors, MRI machines, and patient monitors are all susceptible to interference from electromagnetic emissions. Slip rings used in medical applications must, therefore, meet stringent standards for emission control to prevent such interference.
Key Considerations:
- Electromagnetic Noise: Slip rings must be designed to limit the amount of electromagnetic radiation they emit into the surrounding environment. Excessive emissions can cause “noise” that interferes with nearby devices, resulting in malfunction or incorrect readings.
- Adjacent Device Protection: Medical devices, especially those involved in diagnostic imaging or monitoring, require stable and undisturbed signals. An EMC-compliant slip ring will ensure that the signal integrity of adjacent equipment, such as MRI systems or ECG monitors, is maintained without disruption from the slip ring’s emissions.
Impact on Patient Care:
If a slip ring does not adequately control emissions, it could cause interference that leads to incorrect diagnoses or even medical errors, potentially jeopardizing patient safety.
Immunity Performance
Objective: Ensuring that the slip rings can withstand external interference without affecting device performance.
In addition to controlling emissions, medical slip rings must also be designed to withstand external electromagnetic interference (EMI) from other sources. Hospitals are filled with a variety of devices, including wireless communication systems, power lines, and other medical equipment that may generate electromagnetic fields. A slip ring that is immune to these external sources of interference ensures reliable operation even in these highly electromagnetic environments.
Key Considerations:
- Sources of Interference: Medical equipment, including portable devices and wireless communication systems, can generate external electromagnetic disturbances. A slip ring must be designed to maintain optimal performance even when exposed to radio-frequency interference (RFI), electrical surges, or other types of electromagnetic noise.
- Immunity to Power Surges: Hospitals rely heavily on stable power systems, but power fluctuations and surges are inevitable. Slip rings need to be robust enough to handle these surges without degrading the quality of the signal or causing device malfunctions.
Real-World Scenarios:
- In operating rooms, where wireless devices like handheld surgical instruments or remote monitors are common, a slip ring must remain immune to potential interference from these devices.
- In diagnostic environments, such as MRI rooms, where strong magnetic fields and RF signals are constantly in use, immunity performance is vital to avoid any degradation in imaging quality or the functioning of monitoring equipment.
Impact on Equipment Reliability:
Poor immunity performance can lead to device malfunction, which is particularly concerning in critical medical applications where any disruption can compromise patient safety.
Standards Compliance
Objective: Adhering to internationally recognized standards to ensure that the slip rings meet required EMC levels and offer full regulatory compliance.
To guarantee the quality and safety of medical devices, manufacturers must follow established EMC standards and certifications. Compliance with these standards ensures that the slip rings are not only safe for patient care but also legally acceptable for use in healthcare settings.
Key Considerations:
- IEC 60601-1-2: This is the international standard for the EMC requirements of medical electrical equipment. Compliance with this standard is essential to demonstrate that the slip ring meets the necessary criteria for emission control, immunity, and safety in medical environments.
- FDA EMC Guidelines: The U.S. Food and Drug Administration (FDA) also mandates that medical devices, including slip rings, comply with specific EMC regulations. Manufacturers must ensure that the slip rings they offer are FDA-approved and meet the necessary EMC performance criteria.
- EU Medical Device Regulation (MDR): In Europe, the MDR mandates that medical devices, including their components, meet EMC requirements to ensure they are safe and effective for use in medical settings.
How to Verify Compliance:
- Test Reports: When purchasing a medical slip ring, buyers should request detailed test reports from suppliers that confirm compliance with relevant EMC standards. This documentation can provide peace of mind that the slip ring has undergone the necessary testing and meets international EMC regulations.
- Certification Logos: Certified products will typically display relevant EMC certification logos (such as CE or UL) to show their compliance with global standards. Buyers should always look for these marks as an indicator of quality and safety.
Impact on Legal and Regulatory Compliance:
Failure to ensure that a slip ring meets these EMC standards could result in non-compliance with legal and regulatory requirements, leading to potential recalls, fines, or even the suspension of medical device certifications. Furthermore, it may expose hospitals and medical facilities to liability in case of patient harm resulting from equipment failure.
For medical device buyers, understanding and prioritizing these three key EMC requirements—emission control, immunity performance, and standards compliance—are crucial steps in selecting the right slip rings for their medical applications. Ensuring that slip rings are designed to minimize electromagnetic interference while maintaining immunity to external disruptions will safeguard the integrity of medical equipment and protect patient safety. By focusing on these aspects, healthcare facilities can ensure that their equipment operates smoothly, meets regulatory requirements, and supports optimal patient care.

EMC’s Direct Impact on Patient Safety & Hospital Operations
EMC (Electromagnetic Compatibility) is not just a technical requirement; it directly affects the safety and efficiency of medical devices in a hospital setting. The consequences of inadequate EMC protection can lead to severe disruptions in patient care, equipment malfunction, and legal implications. In this section, we will explore how EMC failures can directly impact patient safety and hospital operations, as well as the broader financial and legal consequences that can arise from non-compliance.
EMI-Induced Errors in Critical Medical Equipment
One of the most significant risks of inadequate EMC in medical devices is the potential for electromagnetic interference (EMI) to cause errors in critical systems. This is particularly concerning for devices like robotic surgery systems, diagnostic imaging equipment, and patient monitoring systems. These devices rely on precise signals and stable functioning to provide accurate readings, perform life-saving procedures, and monitor patients’ vital signs.
Potential Consequences of EMI on Medical Equipment:
- Surgical Robots: Robotic surgery systems, such as those used in minimally invasive procedures, require high precision to ensure patient safety. EMI can disrupt the signals transmitted between the control system and robotic arms, leading to loss of control or erratic movements during surgery. This could result in injury or even death.
- Imaging Systems (MRI/CT Scanners): Magnetic resonance imaging (MRI) and computed tomography (CT) scanners are highly sensitive to electromagnetic interference. If EMI distorts the images produced, it could lead to misdiagnoses, delaying critical treatment or leading to unnecessary procedures.
- Patient Monitoring Systems: These systems continuously track vital signs such as heart rate, blood pressure, and oxygen levels. If EMI interferes with the sensor readings, it may result in inaccurate data being transmitted to healthcare providers. This can lead to incorrect treatment decisions, potentially putting patients at risk.
Case Study Example:
- In one hospital, the robotic surgery system malfunctioned due to EMI from nearby wireless devices used by the surgical team. This caused a delay in the procedure, which resulted in patient complications. The hospital later discovered that their robotic system lacked adequate EMC protection against external interference, leading to costly repairs and lost trust in their surgical capabilities.
Cost of Downtime vs. Investing in Compliant Slip Rings
The financial cost of downtime due to EMC-related issues is substantial for any healthcare facility. When medical equipment fails or experiences interruptions due to poor EMC performance, it can cause delays in patient care, reduced productivity, and even financial penalties for non-compliance with healthcare regulations.
Key Costs Associated with EMC Failures:
- Service Interruptions: If critical medical equipment such as MRI machines, patient monitors, or robotic surgery systems are impacted by EMI, the hospital may need to take these devices offline for repairs or recalibration. In high-demand environments, this downtime can significantly disrupt the hospital’s ability to provide care, affecting patient wait times and hospital efficiency.
- Replacement Costs: In some cases, EMC failure could lead to hardware damage or the need to replace key components of medical devices, such as slip rings. The cost of replacement is not only high but can also result in operational delays as devices are removed from service for maintenance.
- Reduced Productivity: As medical staff may have to spend extra time troubleshooting and handling equipment failures, the overall productivity of hospital personnel declines. This can lead to slower patient throughput, longer wait times, and a decrease in the number of procedures performed.
- Increased Operational Costs: Downtime and equipment failures also drive up operational costs. Hospitals may need to employ temporary or replacement equipment, incur repair costs, and allocate additional resources to handle the impact of EMC-related disruptions.
ROI of Investing in Compliant Slip Rings: Investing in slip rings that meet EMC standards may come at a higher initial cost, but the long-term ROI is significant. By preventing the costly consequences of EMI, hospitals can ensure that their equipment operates reliably, reducing downtime, repair costs, and ensuring smoother patient care. Hospitals can also avoid potential legal risks and fines associated with regulatory non-compliance, further strengthening the financial case for investing in high-quality, EMC-compliant slip rings.
Legal and Regulatory Implications of EMC Failures
Medical devices, including their components like slip rings, must adhere to strict regulatory standards to ensure that they are safe and effective for use in patient care. Failures to comply with EMC regulations can lead to serious legal consequences, including product recalls, legal liability, and fines.
Regulatory Risks:
- FDA Recalls: If a medical device fails to meet EMC standards, the U.S. Food and Drug Administration (FDA) may issue a recall to protect patients. A recall due to EMC failure can damage the hospital’s reputation and cause a significant financial loss, as well as administrative costs associated with removing non-compliant devices from service.
- EU MDR Non-Compliance: In the European Union, the Medical Device Regulation (MDR) mandates that devices meet specific EMC requirements. Non-compliance with these standards could result in a ban on the device in the market, legal action, and loss of market trust.
- Liability in Case of Patient Harm: If poor EMC performance results in patient injury, death, or worsened conditions, the hospital may face liability lawsuits. Patients or their families may seek compensation for damages caused by the malfunctioning of medical equipment. Legal battles can be long and costly, further straining the hospital’s financial and operational resources.
Example:
- A hospital in Europe faced legal consequences when a series of patient monitoring systems malfunctioned due to electromagnetic interference from nearby devices. The failure to meet EMC requirements led to misreadings of vital signs, resulting in a patient’s deteriorating condition. The hospital had to recall the affected devices, pay fines, and face a lawsuit from the patient’s family. This situation highlighted the importance of ensuring that all medical equipment adheres to EMC standards to avoid legal and financial repercussions.
Impact on Hospital Reputation and Trust
The reputation of a hospital is closely tied to the reliability of its equipment and the quality of patient care it provides. Frequent equipment failures, especially those caused by poor EMC performance, can erode trust among both patients and healthcare professionals.
Reputation Damage:
- Patient Confidence: When medical devices experience failures due to EMI, it undermines patients’ confidence in the hospital’s ability to provide safe and effective care. A hospital known for unreliable equipment may see a drop in patient numbers, which can have long-term effects on its financial stability.
- Professional Trust: Healthcare professionals depend on medical equipment to provide accurate diagnoses and treatments. If doctors and nurses begin to lose trust in the reliability of a hospital’s equipment, they may seek alternative facilities with better-quality, EMC-compliant devices, leading to a loss of skilled staff and a decline in service quality.
EMC is a crucial factor in ensuring the safety and effectiveness of medical equipment. Failures in EMC can have far-reaching consequences, including equipment malfunctions, increased costs due to downtime, legal liabilities, and significant damage to a hospital’s reputation. By prioritizing EMC compliance in the selection of medical slip rings, hospitals can mitigate these risks, ensuring smooth operations, patient safety, and long-term financial and legal security. Compliance with EMC standards is not just a technical necessity; it’s a vital aspect of maintaining high-quality care and safeguarding the hospital’s operational success.

How to Evaluate EMC Claims When Purchasing
Evaluating Electromagnetic Compatibility (EMC) claims from suppliers is a crucial part of the purchasing process for medical slip rings. Since EMC plays a critical role in ensuring the safety and proper functioning of medical equipment, it’s essential that healthcare facilities and purchasing teams carefully assess the reliability and validity of EMC claims before making a purchase. This section will guide you on how to effectively evaluate these claims and make an informed decision.
Supplier Checklist: Key Documentation and Information
When assessing a supplier’s EMC claims, it’s important to request specific documentation and information to verify that the slip ring will perform according to EMC standards. Here are the key items to check:
- Third-Party Testing Documentation
A credible supplier should provide independent third-party test reports that demonstrate the slip ring’s compliance with relevant EMC standards. These reports should include:- Testing Methods: Information on the testing environment and methods used to evaluate EMC performance (e.g., IEC 60601-1-2, ISO 13485).
- Test Results: Detailed results showing the slip ring’s emissions and immunity levels compared to the required limits.
- Testing Facility Credentials: The certification of the testing facility, ensuring that it is accredited to perform EMC testing.
Why It’s Important: Third-party testing ensures impartiality and trustworthiness. Internal test claims made by the supplier may not be as reliable as independent verification from an accredited laboratory.
- Customization Options for Specific Medical Applications
Every medical facility has unique requirements depending on its devices, room layouts, and specific equipment configurations (e.g., MRI rooms, ORs). Ask the supplier about their ability to customize slip rings to meet these specific needs.- Application-Specific Adjustments: Can the slip ring be tailored for environments with high levels of electromagnetic interference (EMI) or unique constraints (such as temperature and space limitations)?
- Device Compatibility: Ensure the supplier can confirm that their slip rings are compatible with the specific medical equipment in use at your facility, such as robotic surgery systems or imaging devices.
Why It’s Important: Standard slip rings may not be suited to highly sensitive environments, so customization ensures that your facility’s specific needs are met.
- Long-Term EMC Stability Data (Aging, Temperature Effects)
EMC performance is not always static over time. It’s important to assess how the slip ring will perform under varying conditions, especially over its expected lifespan.- Aging and Wear: How does the slip ring’s EMC performance degrade with age and usage? Does it still meet EMC standards after years of operation?
- Temperature and Environmental Stability: Will the slip ring maintain its EMC compliance in extreme temperatures or varying environmental conditions commonly encountered in healthcare settings?
Why It’s Important: Medical equipment is often used continuously or in high-stress environments, so long-term stability is crucial for maintaining compliance and ensuring uninterrupted operations.
- Maintenance and Post-Installation Support
Ask the supplier about their post-installation support, including the availability of service teams to address any potential EMC-related issues that may arise after installation.- EMC Support: Can they provide on-site support if EMC issues occur, or do they offer tools to assess EMC performance during routine maintenance?
- Monitoring Services: Do they offer monitoring or diagnostic tools to detect EMC issues before they affect device performance?
Why It’s Important: Even compliant equipment can face unforeseen EMC challenges after installation, and ongoing support helps resolve issues promptly to avoid disruptions in medical operations.
Red Flags: What to Watch Out For
While evaluating EMC claims, there are several warning signs that may indicate a supplier is overstating or misleading about the capabilities of their slip rings. Be vigilant for the following red flags:
- Vague “EMC-Friendly” Marketing Language
Some suppliers may use non-specific terms like “EMC-friendly,” “low noise,” or “compliant with standards” without providing concrete evidence or details. These terms are often used as a marketing tactic and are insufficient without supporting documentation.What to Do: Always ask for specific test reports, certification information, and performance data. If the supplier cannot provide these, consider looking elsewhere. - No Independent Test Reports
Suppliers who claim to meet EMC standards but fail to provide independent, third-party test results should be scrutinized carefully. In-house testing results are not always reliable, as they might lack transparency and objectivity.What to Do: Request third-party test results, as independent testing is a critical validation of the supplier’s claims. - Failure to Meet Relevant Certifications
If a supplier cannot provide evidence that their slip rings comply with key industry standards, such as IEC 60601-1-2, FDA guidelines, or EU MDR regulations, this is a major red flag. Non-compliance with established standards poses significant risks to both patient safety and legal compliance.What to Do: Verify that the supplier’s slip rings comply with recognized certifications, and request certification numbers or copies of documents to confirm their validity. - Unclear or Unspecified Test Conditions
If the supplier provides test reports but the conditions under which the tests were conducted are vague or unspecified, it’s difficult to assess the real-world applicability of the results. For example, if tests were conducted in ideal conditions but not in environments similar to your hospital’s, the results may not be representative of actual performance.What to Do: Ask for clarification on testing environments and conditions. Ensure that the results reflect the typical operating conditions for your medical facility, such as EMI levels in the operating room or proximity to MRI equipment. - Overly Cheap or Unreasonably Expensive Products
While budget constraints are common in healthcare procurement, be cautious of suppliers who offer extremely low-priced slip rings that claim full EMC compliance. On the other hand, excessively high prices should also raise concerns. There is a balance to be found between quality and cost.What to Do: Compare prices with similar suppliers and request a breakdown of the costs. A competitive price range should match the level of technology, customization, and compliance the product offers.
Questions to Ask Suppliers
To ensure you are making an informed decision, it’s important to ask the right questions during the purchasing process. Here are key questions to ask potential suppliers about their EMC claims:
- “Can you provide third-party testing documentation for the slip ring?”
- This will help confirm the slip ring’s compliance with relevant EMC standards.
- “What standards does this slip ring meet, and how can we verify its compliance?”
- Ensures the slip ring meets the necessary regulatory requirements.
- “Can you customize the slip ring for our specific hospital environment, such as MRI rooms or operating rooms?”
- It helps custom slip rings to meet the unique EMC needs of your facility.
- “How does the slip ring perform under long-term use, and can you provide data on aging and temperature effects?”
- Confirms the slip ring’s reliability over time in your facility’s operating conditions.
- “What kind of post-installation support do you offer if EMC issues arise?”
- Ensures that any future issues can be addressed promptly to avoid disruptions.
Evaluating EMC claims when purchasing medical slip rings requires thorough investigation and due diligence. By asking the right questions, checking for third-party documentation, and being vigilant about vague marketing or unsubstantiated claims, healthcare purchasers can make more informed decisions. Prioritizing reliable and transparent suppliers will help ensure that the slip rings selected meet the necessary EMC standards, ultimately safeguarding patient safety, minimizing operational downtime, and ensuring compliance with relevant regulations.
Emerging Trends Affecting Buyer Decisions
As the medical field continues to evolve, new technologies, shifting regulations, and changing patient care needs are influencing how healthcare providers approach the procurement of medical equipment, including slip rings. Buyers must stay informed about these emerging trends to ensure that their investments in equipment like slip rings are future-proof, compliant, and optimized for patient care. In this section, we will explore the key trends that are impacting buyer decisions when selecting EMC-compliant medical slip rings.
Wireless Medical Devices & Higher EMC Challenges
Trend: The increasing reliance on wireless medical devices is placing greater pressure on the electromagnetic environment within hospitals. These devices, ranging from handheld diagnostic tools to implantable devices, are becoming integral to patient care. However, their widespread use introduces new challenges in terms of electromagnetic interference (EMI) that must be managed effectively.
Impact on EMC:
- Increased EMI Risks: Wireless devices emit radio-frequency signals that can interfere with sensitive medical equipment. For instance, devices like wireless infusion pumps, telemetry systems, or portable patient monitors can generate electromagnetic fields that disrupt the functioning of other hospital equipment, such as MRI scanners or surgical robots.
- Demand for Stricter EMC Compliance: As the number of wireless devices increases, the demand for medical slip rings that offer stronger immunity against interference and better emission control will rise. These slip rings must be able to coexist with an increasingly crowded electromagnetic environment, ensuring that both wireless and wired devices can function without interference.
Consideration for Buyers:
- Buyers must ensure that any slip rings purchased are capable of meeting stricter EMC standards that accommodate wireless devices. These slip rings should be designed to minimize the risk of cross-device interference and support the growing number of wireless medical technologies in use.
AI-Driven Diagnostics Requiring Ultra-Low Noise
Trend: Artificial intelligence (AI) is revolutionizing healthcare by enabling faster and more accurate diagnostics, personalized treatment plans, and real-time monitoring. However, AI-driven systems—especially those used for high-precision diagnostics, such as in imaging or robotics—require extremely low noise levels to function effectively.
Impact on EMC:
- Demand for Ultra-Low Noise: AI algorithms, particularly in fields like medical imaging (e.g., MRI, CT scanner) and robotic surgery, are highly sensitive to electromagnetic interference. Even minor EMI can distort data, affecting the performance of AI systems, and potentially leading to incorrect diagnoses or robotic malfunctions.
- Precision and Reliability: AI-powered devices rely on the precise and accurate transmission of data. Any EMI interference in the communication signals between components can compromise the reliability of these systems, making it critical for slip rings to provide consistent and ultra-low noise performance.
Consideration for Buyers:
- Healthcare buyers need to ensure that the slip rings selected for AI-driven systems are capable of handling low-noise environments and offering robust EMC protection. A slip ring that provides better noise suppression will directly impact the reliability of AI systems, ensuring that healthcare professionals can make accurate, data-driven decisions.
Green Hospitals: Energy Efficiency vs. EMC Balance
Trend: The global push for sustainability is influencing healthcare facilities to adopt greener practices. This includes the adoption of energy-efficient technologies, waste reduction, and environmentally friendly building designs. Medical equipment and components, including slip rings, must align with these sustainability goals without compromising on performance.
Impact on EMC:
- Energy-Efficient Technologies: Green hospitals are increasingly looking to reduce energy consumption by utilizing low-power and energy-efficient technologies. While these technologies help lower operating costs and environmental impact, they may also face challenges in managing electromagnetic compatibility.
- EMC Considerations in Green Technologies: As hospitals embrace energy-efficient equipment, some slip rings may need to be optimized for lower power consumption while maintaining high EMC performance. The design of these slip rings must balance power efficiency with the need for strong electromagnetic shielding and immunity, which can sometimes conflict in high-performance environments.
Consideration for Buyers:
- Buyers must weigh the benefits of energy efficiency against the need for high-quality EMC performance. They should seek slip rings that not only support green initiatives but also maintain EMC compliance to ensure that performance and patient safety are not compromised.
The Increasing Complexity of Hospital Environments
Trend: As hospitals grow larger and more complex, with expanding departments and new medical technologies, the electromagnetic environment becomes more challenging to manage. The installation of multiple medical devices, each with its own EMC requirements, creates an environment where EMI risks are more pronounced.
Impact on EMC:
- Dense Electromagnetic Environments: The introduction of more medical equipment, including advanced imaging machines, surgical robots, and wireless monitoring devices, increases the likelihood of electromagnetic interference. Ensuring that these devices function properly together in a shared environment requires careful consideration of the EMC performance of all components, including slip rings.
- Need for Tailored Solutions: As the hospital environment becomes more complex, a “one-size-fits-all” approach to EMC protection becomes less viable. Hospitals may need tailored solutions, such as customizable slip rings that are designed to mitigate specific electromagnetic challenges in each department (e.g., radiology, ICU, or operating rooms).
Consideration for Buyers:
- In such complex environments, healthcare buyers should consider purchasing slip rings that can be customized to meet the specific EMC needs of different hospital departments. By tailoring the EMC solution to each environment, hospitals can ensure seamless operations and prevent interference between various medical devices.
Regulatory Updates and Stricter Compliance Requirements
Trend: As the medical industry continues to evolve, so too do the regulations governing medical devices. New or updated standards related to EMC, patient safety, and device interoperability are emerging globally, including revisions to standards like IEC 60601-1-2, FDA guidelines, and EU Medical Device Regulations (MDR).
Impact on EMC:
- Tighter Regulatory Oversight: Regulatory bodies are imposing stricter compliance requirements for medical equipment, including slip rings, to ensure that devices meet the highest safety and performance standards. Compliance with the latest EMC standards is essential for manufacturers to market their products in regions like the U.S. and Europe.
- Global Compliance Variability: Different regions may have varying EMC standards, and manufacturers will need to meet the specific requirements for each market. This could complicate the purchasing decision for hospitals that operate internationally or are subject to multiple regulatory frameworks.
Consideration for Buyers:
- Buyers must stay updated on regulatory changes to ensure that the slip rings they purchase are compliant with the latest standards. Inquire about how the supplier ensures compliance with global standards and request documentation verifying that the slip rings meet the necessary EMC certifications for your region.
Emerging trends in healthcare are shaping the future of EMC compliance in medical devices, including slip rings. With the rise of wireless medical devices, AI-driven diagnostics, green hospitals, and increasingly complex hospital environments, buyers must prioritize EMC performance when making purchasing decisions. Additionally, staying informed about regulatory changes is crucial to ensure compliance and avoid potential risks. As these trends evolve, healthcare facilities must work closely with suppliers to ensure that their equipment remains reliable, safe, and compatible with the latest technological advancements, all while balancing performance, sustainability, and compliance.
Partnering with the Right Manufacturer
When it comes to selecting the right slip ring for your medical devices, partnering with a trusted and reliable manufacturer is essential. At Grand Slip Ring, we understand that the quality and performance of your medical equipment depend heavily on the components that power them, including the slip rings. In this section, we’ll outline the key questions to ask and the reasons why partnering with a manufacturer like Grand Slip Ring can provide you with the EMC-compliant solutions that you need.
Key Questions to Ask When Choosing a Slip Ring Supplier
Choosing a supplier isn’t just about purchasing a product; it’s about building a long-term partnership. At Grand Slip Ring, we are committed to ensuring that your needs are met not only during the purchase but throughout the entire lifespan of the product. To make sure you are getting the right product and support, here are key questions we recommend asking when evaluating a slip ring supplier:
- “Can you simulate our hospital’s EMI environment during testing?”
This question ensures that the slip ring will be tested in a simulated environment that closely resembles the electromagnetic conditions in which it will be used. At Grand Slip Ring, we offer customized testing scenarios based on your hospital’s unique electromagnetic challenges, ensuring that the slip ring will perform reliably in your specific setting. - “What certifications and EMC standards do your slip rings meet?”
Understanding which EMC standards the supplier complies with is crucial for legal and regulatory compliance. At Grand Slip Ring, we guarantee that our products meet international EMC standards such as IEC 60601-1-2, FDA EMC guidelines, and EU Medical Device Regulations (MDR). We provide full documentation to validate these certifications, ensuring that you are always in compliance. - “Can you provide long-term EMC stability data, including performance after aging and exposure to temperature variations?”
EMC performance is not static; it can degrade over time. At Grand Slip Ring, we offer long-term performance data for our slip rings, demonstrating how they maintain EMC compliance even after years of use, under varying environmental conditions like temperature and humidity. This ensures that your equipment will continue to perform reliably over time. - “What kind of post-installation support do you offer?”
Post-installation support is essential to address any potential EMC-related issues that might arise after the slip ring is installed. At Grand Slip Ring, we don’t just sell you a product and walk away. Our dedicated support team is always ready to assist with troubleshooting, maintenance, and resolving any EMC-related concerns that may surface after installation.
Why Partner with Grand Slip Ring?
Choosing the right manufacturer goes beyond just purchasing a product; it’s about the ongoing relationship you build. At Grand Slip Ring, we stand out in the industry for several reasons that ensure we are the right partner for your medical equipment needs.
- Expertise in Medical EMC Compliance
We specialize in providing slip rings designed to meet the stringent EMC requirements of the medical industry. Whether you need slip rings for surgical robots, patient monitoring systems, or diagnostic imaging equipment, we understand the unique challenges that come with medical environments and ensure our products meet the highest standards for EMC performance. - Customized Solutions for Complex Needs
Every medical facility is different, and we understand that one-size-fits-all solutions are not always the best approach. At Grand Slip Ring, we offer highly customizable slip rings tailored to meet your hospital’s specific EMC requirements. Whether it’s a unique electromagnetic environment in your operating room or a need for ultra-low noise in imaging equipment, we work with you to deliver the best solution. - Long-Term Reliability and Support
The performance of medical equipment can have direct consequences on patient safety and care. At Grand Slip Ring, we don’t just focus on meeting the immediate EMC standards; we ensure that our slip rings will continue to perform reliably over time. Our commitment to long-term stability is backed by data and supported by our team, who are ready to provide ongoing assistance. - Global Compliance and Certification
As a globally recognized brand, we understand the importance of regulatory compliance in different regions. Our slip rings meet global standards, including IEC 60601-1-2, FDA EMC guidelines, and EU MDR. We provide full transparency, offering detailed certification documentation so you can have peace of mind knowing that the products you purchase are fully compliant. - Proven Track Record in Medical Applications
With years of experience in the medical sector, we have worked with leading healthcare providers to deliver reliable and compliant EMC solutions. Our expertise and track record make us a trusted partner for hospitals, clinics, and medical equipment manufacturers seeking to ensure the safe and efficient operation of their devices.
Success Story: A Hospital’s Improved EMC Performance with Grand Slip Ring
We have seen firsthand the positive impact our slip rings have had on hospitals and medical centers. For example, one of our hospital clients was facing frequent interference issues with their robotic surgery systems, causing delays in procedures and potential risks to patient safety. After working closely with their team, we customized a slip ring solution that met their specific EMC requirements. The result? A dramatic reduction in electromagnetic interference, leading to smoother operations and improved patient outcomes. The hospital was able to trust their robotic systems again, knowing they were supported by a reliable, EMC-compliant slip ring solution from Grand Slip Ring.
Partnering with the right manufacturer for your medical slip ring needs is a critical decision that directly impacts patient safety, device performance, and hospital operations. At Grand Slip Ring, we are committed to providing high-quality, EMC-compliant solutions that ensure the reliable operation of medical equipment. Our expertise, customizable solutions, and long-term support make us the trusted partner you can count on to meet your facility’s EMC requirements. By choosing Grand Slip Ring, you’re not just purchasing a product—you’re investing in a lasting partnership that prioritizes quality, compliance, and patient care.